Noze Hardware Test Automation Platform
Designed and deployed a full-stack hardware-in-the-loop (HIL) automation platform to support verification and validation (V&V) of breath-based diagnostic devices. The system enabled repeatable, high-quality dataset generation by tightly controlling physical conditions, sensors, and experiments.
Hardware-in-the-Loop System for Medical Device Validation

Category
professional
Company
Noze
Status
Completed

Why we built it
Medical devices live and die by data quality.
At Noze, teams across hardware, product, and applied ML needed large volumes of repeatable, reliable, and traceable sensor data to validate designs, test hypotheses, and support regulatory submissions. Manual testing and ad‑hoc rigs could not scale, and they introduced variability that undermined confidence in results.
The goal was clear: build an automated system that could reliably generate ground‑truth datasets under controlled conditions, at scale, while meeting the expectations of regulated medical development.
What I built
I designed and built a complete HIL automation platform from the ground up.
This was true full‑stack hardware work:
- Automation software written in Python to orchestrate experiments, control hardware, and manage data collection
- Sensor integration across multiple OEM sensing modalities
- Actuator control for gas flow, pressure, humidity, and environmental conditions
- Firmware and experiment logic to coordinate timing, calibration, and state transitions
- Data pipelines to ingest raw sensor streams, annotate metadata, and structure datasets
- Electrical systems including custom wiring, soldering, and signal integrity considerations
- Mechanical and pneumatic design to ensure physical isolation, repeatability, and non‑interference between subsystems
The result was a scalable validation plant capable of running thousands of controlled experiments with minimal human intervention.
System flow (experimental plant)
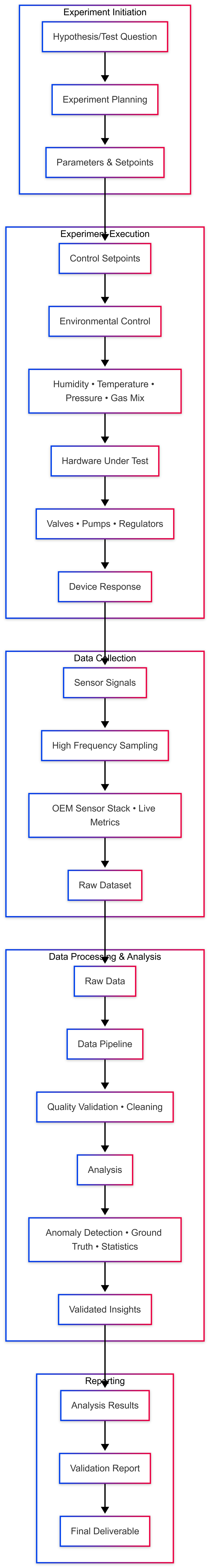
Outcomes
- Validated 10,000+ sensor datasets: Across 25 automated HIL rigs, supporting 5 iterations of the flagship device.
- Tripled testing throughput: While reducing cost per datapoint by 40%.
- Regulatory Compliance: Enabled traceable, audit‑ready datasets supporting ISO/FDA submissions.
Precision as a Requirement
I learned what it actually means to generate datasets for regulated industries: data is only valuable if it is repeatable, explainable, and defensible. Automation is not about speed alone; it is about removing ambiguity and human variability from the system.
Bridging Engineering and Science
Because all work was driven by internal teams, I spent significant time translating vague hypotheses into executable experiments. That meant understanding what teams were trying to prove, designing the right physical conditions to test it, and building tooling that could surface failures, drift, and edge cases early.
Treating test infrastructure as a first‑class product accelerates engineering, de‑risks decisions, and creates trust in both the data and the product built on top of it.
Technologies Used
Key Outcomes
- •Validated 10,000+ sensor datasets across 25 rigs
- •Tripled testing throughput while reducing cost per datapoint by 40%
- •Enabled ISO/FDA compliant documentation for device approval